Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


Procuring adequate vaccine quantities and safely deploying COVID 19 vaccines to India’s population of over 1.3 billion people once the vaccines are available, is going to be an unprecedented administrative and logistical challenge. However, with a clear strategy and phased, decentralized delivery, it should be possible to vaccinate 80% of India’s population by December 2021.
In preparation, several countries are currently finalizing and communicating their vaccine deployment plans. Examples:
Given the scale and the stakes involved, India needs to take some decisive steps to ensure its people are vaccinated in a timely manner. The world’s eyes are already on India as the country is the world’s biggest vaccine manufacturer and a major contributor to reliable vaccines. India can provide quality, low-cost vaccines not only for internal use but also for global purposes.
As mentioned earlier, it should be possible to vaccinate 80% of India’s population by December 2021.
But why 80%?
Well, hitting the ambitious target of eighty percent within the short time can ensure that Herd immunity is achieved through the vaccination exercise; as it is still unknown for how long the vaccine conferred immunity will work.
Herd immunity works only when a large subset of the population is concurrently immune to the disease. As an example, if the vaccine conferred immunity lasts one year but vaccine distribution takes three years, the objective of achieving herd immunity cannot be met. Therefore, it is critical that vaccination be implemented in as short a time frame as possible.
|
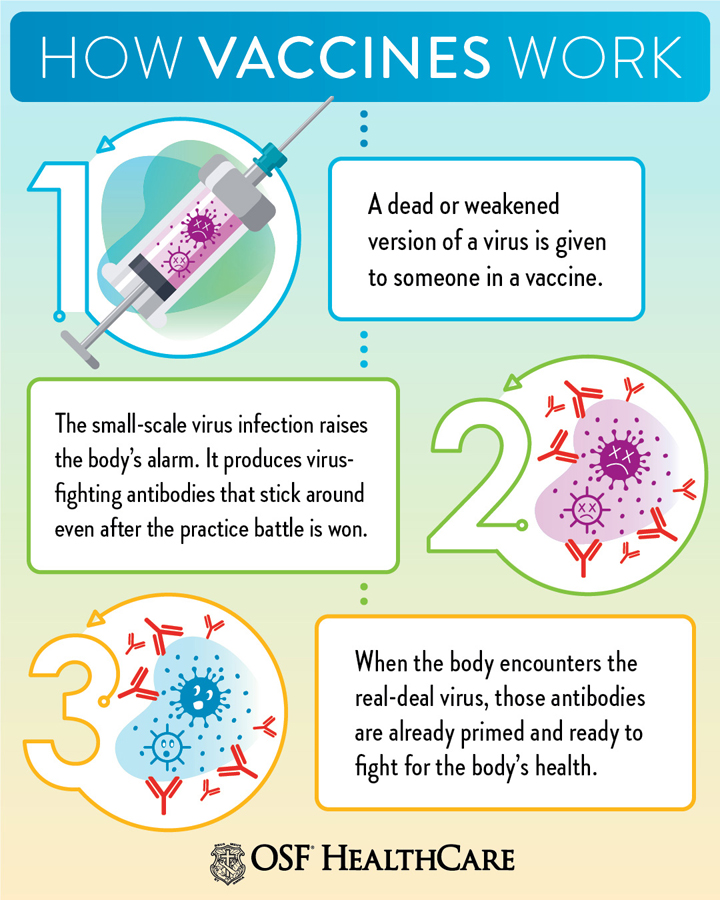
What is Herd immunity? ‘Herd immunity’, also known as ‘population immunity’, is a concept used for vaccination, in which a population can be protected from a certain virus if a threshold of vaccination is reached. For example, if 80% of a population is immune to a virus, four out of every five people who encounter someone with the disease won’t get sick (and won’t spread the disease any further). In this way, the spread of infectious diseases is kept under control. Depending on how contagious an infection is, usually 50% to 90% of a population needs immunity to achieve herd immunity. When most of a population is immune to an infectious disease, this provides indirect protection— or herd immunity to those who are not immune to the disease. Achieving herd immunity with safe and effective vaccines makes diseases rarer and saves lives. |
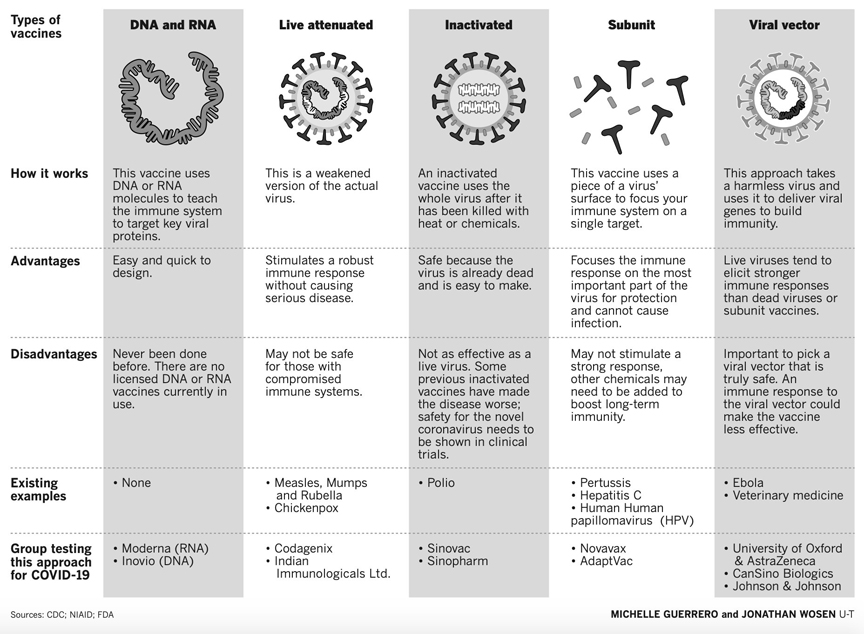
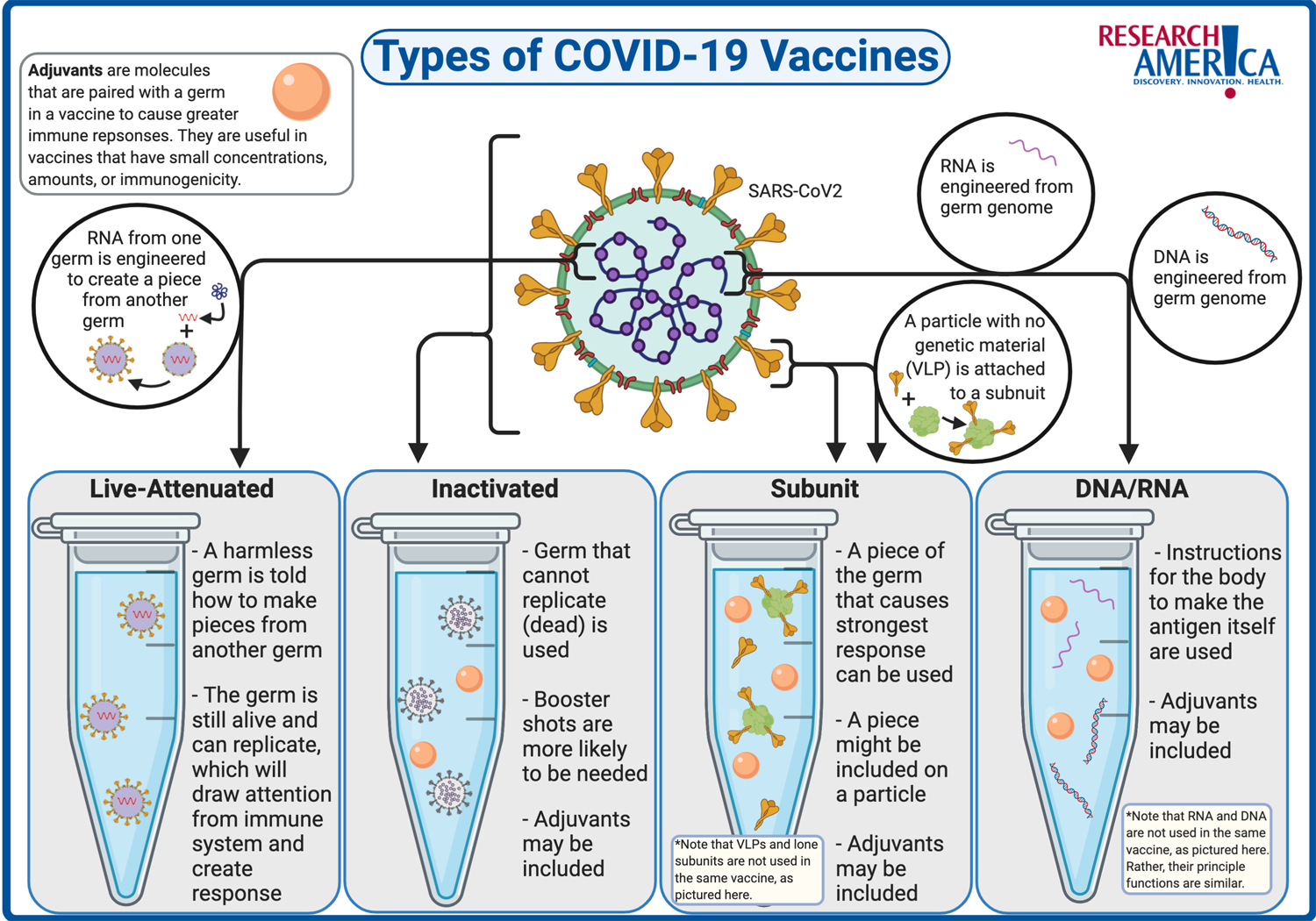
India currently is one of the leading manufacturers and suppliers of vaccines in the world. It solely accounts for around 60% of the total vaccines supplied to the UNICEF. The cost of manufacturing and clinical trials in India is relatively lower than in developed countries. Moreover, technological advancements and improved cold chain storage facilities have led to increased vaccine production capacity in the country - International Market Analysis Research and Consulting Report.
Since India has emerged as one of the significant vaccine manufacturing hubs of the world, the vaccine for COVID-19 may be developed anywhere in the world, but the production of required quantities may not be feasible without the involvement of Indian manufacturers.
India currently produces nearly 120 million doses of vaccines per month under the Universal Immunisation Programme (UIP). COVID 19 vaccine on the other hand needs to reach one billion people —nearly 16 times the annual recipients under UIP.
Even India’s well oiled and successful UIP machinery, in its current state, will be inadequate to carry out the COVID 19 vaccination exercise within a short time period.
India certainly has the technological expertise to ramp up manufacturing of vaccines. But the government needs to facilitate this scaling-up the process through regulatory and, financial interventions.
An important facet to balance in this equation is making the vaccine available to Indians at an affordable price, while also catering to the global requirement. This is also an opportunity for India to strengthen strategic ties with partnering countries by providing COVID-19 vaccines at affordable rates.
However, all these opportunities come at the cost of ramping up vaccine supply – a necessity India needs to address immediately.
On the supply side, it is unlikely that the two billion doses India needs will be available immediately upon approval of vaccines. Thus, vaccine distribution will have to be phased to match the available supply.
Additionally, vaccine deployment will need to account for several challenges such as transport and storage capacities, need for trained people to take recipient consent, a way to monitor who has received the vaccine, which vaccine has been administered, and track adverse reactions, if any.
In the face of a supply crunch, India will have to prioritise vaccine recipients in the initial stages to ensure that critical sub-populations are covered first. However, prioritisation strategy cannot be a substitute for poor capacity. It is a temporary arrangement till increased production capacity meets vaccine demand.
Rationale behind prioritization: Although everyone is affected by the COVID-19 pandemic, its impact is not shared equally. Some groups are experiencing serious illness and death at higher rates specifically associated with biological factors, e.g. those who are older or have underlying health conditions. Other groups are experiencing disproportionately greater health and other burdens because of societal factors, e.g. limitations of people living in poverty to practice physical distancing and experiencing barriers to accessing quality health care.
As more vaccine candidates get regulatory approvals, the supply demand gap will shrink. Once critical sub populations are vaccinated, no further prioritisation will be required. Prioritisation can be done through two broad approaches: Essential first and Demography first.
Individuals engaged in occupations of utmost importance to society and who have a high degree of exposure to COVID- 19 as a result of their occupation are accorded highest priority for vaccination. This will include those who are at the forefront of managing the pandemic (healthcare workers, sanitation workers, etc.) and those who work to ensure business continuity of routine services (utility workers, logistics workers, police, etc.)
This strategy categorizes individuals based on their risk of exposure to COVID-19 and the probability of requiring hospitalization. This approach prioritizes individuals over 65 years or with co-morbidities to get the vaccine first, followed by randomization.
The guiding principle of national equity is to ensure that there is equitable access to vaccines, and that
Groups at increased risk of COVID-19, due to underlying societal, geographic or biomedical factors, benefit from vaccination.
“Because vaccine supply will be limited at first, the decisions about who gets those first doses could save tens of thousands of lives.”
An increase in vaccine manufacturing capacity is imperative to meet the vaccine demand. Securing vaccine supply would include regulatory approval of vaccines for use in India, tying up with vaccine research institutions and increasing manufacturing capacities to align with demand requirements.
Factors under consideration
Cost: Current reports suggest that the Moderna vaccine may cost about INR 1,125 per dose, while the Oxford vaccine may be available at a cheaper cost of INR 225. Waiting longer for a cheaper vaccine, will incur high social and economic costs.
Supply chain requirements: nucleic acid-based vaccines would require cold chains of extremely low temperatures and deploying them across the country would not be feasible.
Delivery method: Injectable or nasal. Delivering injectable vaccines would require development of a trained workforce.
Regarding Pricing
There are various ways for the government to intervene in the vaccine pricing.
Way 1: Let market forces determine the price. The advantage of this approach is that more vaccines would be incentivized to be brought into the market leading to competitive pricing. Households would bear the entire cost of the vaccine, which would lead to inequitable distribution and exclusion.
However, without any government intervention, it would be difficult to achieve herd immunity at practical prices.
Way 2: Government can bear all costs of the vaccine, effectively making it zero price for Indian residents since vaccination is a public good. However, this approach can distort the market and increase chances of pilferage and corruption. Moreover, there is a significant opportunity cost for the money the government will spend on vaccinating everyone, including those who can afford the vaccine.
Therefore, the best approach is allowing market price for the vaccine plus a direct benefit transfer for those who are unable to afford the vaccine.
This approach will lower the immediate cost of roll out, target subsidy to the needy and well off households pay fair share. This will also lower the burden on government funding while ensuring those who cannot afford the vaccine, can get access to it.
Distribution at India’s scale is a non-trivial planning and management challenge. Solving it requires the ability to quickly mobilize nation-wide decentralized administrative machinery in a phased manner.
Solutions
Vaccination booths
Vaccination booths can be set up within each targeted geographical area with a well-documented set of procedures and appropriate personnel.
Hub spoke Distribution Model
In this the government procures the vaccines and distribution is done by a variety of private and public agencies such as private hospitals, primary health centres, and the armed forces medical corps. The government can contract out distribution district-wise to these agencies.
Demand- driven distribution
This market based model can be run by private hospitals, pharmacies and medical practitioners.
As the secured supply increases, the government can allow people the choice to get vaccinated at private medical centres. This is similar to vaccinating children at private clinics and hospitals outside the government-run Universal Immunization Programme.
The vaccine roll-out will need to be monitored to ensure correct number of doses are given to each individual, detect any adverse events and assess efficiency of the vaccine.
If the vaccine recipient status of an individual is not tracked, it is possible that individuals are inadvertently administered excess doses. Also, if the vaccine is found to ineffective or faulty after it has been administered, a need can arise to reach the correct vaccine recipients and take corrective action.
This necessitates the formation of a database to document vaccine recipients, vaccine dose and adverse events. As deployment progresses, data from vaccine performance, approval of new vaccines, changes in vaccine storage conditions, etc. should be used to alter the deployment strategy.
Note: The database implementation should be in compliance with “privacy by design” principles.
Rapid and wide scale deployment of a COVID-19 vaccine is a challenging task of unprecedented proportion. However, with a clear strategy and phased, decentralized delivery, the target of vaccinating the population is achievable.
This would require investment and urgent action from the Indian government, participation of the private sector and clear engagement with the public. India’s capabilities of producing low-cost, reliable vaccines should be leveraged.
Further, India should invest in training health workers, and set up a robust and transparent post-market surveillance capability for monitoring vaccine performance.
Courtesy: Indian Public Policy Review 2020
© 2024 iasgyan. All right reserved