Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


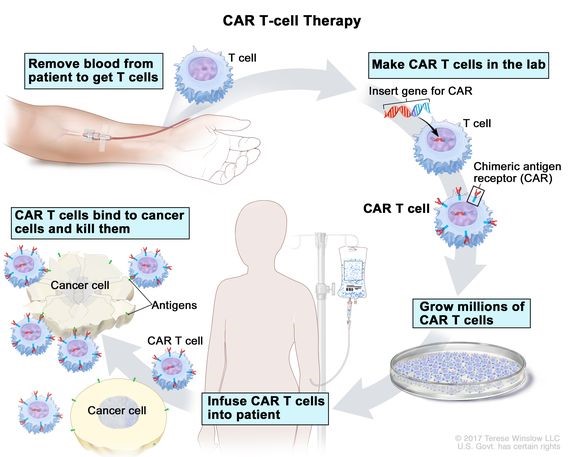
Source: Cancer.gov
Disclaimer: Copyright infringement not intended.
Context
Details
About CAR-T Cell Therapy
Mechanism of Action:
|
Introduction to T Cells
Types of T Cells:
|
Development and Manufacturing Process:
Clinical Applications:
Side Effects:
About Cancer
Types of Cancer:
Causes and Risk Factors:
About Gene Therapy
Types of Gene Therapy:
Steps in Gene Therapy:
Applications of Gene Therapy:
Challenges:
Conclusion
Sources:
|
PRACTICE QUESTION Q. Continued research and clinical development are essential to unlock the full potential of CAR-T cell therapy. Discuss. (250 Words) |
© 2024 iasgyan. All right reserved