Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now



Source: PIB
Disclaimer: Copyright infringement not intended.
Context
Details
Chaperones
Classification of Chaperones
Chaperones can be classified into several major families based on their structure, function, and mechanism of action:
Functions of Chaperones
Key Single Molecule Techniques
|
Technique |
Principle |
Applications |
Advantages |
Limitations |
|
Atomic Force Microscopy (AFM) |
Measures forces between a sharp probe and sample surface |
Imaging surfaces, measuring mechanical properties |
High resolution, versatile |
Limited to surface studies, slow scanning speed |
|
Optical Tweezers |
Uses laser beams to trap and manipulate small particles |
Studying molecular motors, force measurements |
High precision in force measurements |
Requires high laser power, potential photodamage |
|
Single-Molecule Fluorescence (SMF) |
Detects fluorescence from individual molecules |
Tracking molecular dynamics, interactions |
High sensitivity, real-time observation |
Photobleaching, background fluorescence |
|
Magnetic Tweezers |
Uses magnetic fields to apply force to magnetic particles |
Studying DNA-protein interactions, molecular mechanics |
Can apply force over a wide range, low photodamage |
Limited to magnetic materials, less precise than optical tweezers |
|
Patch-Clamp Technique |
Measures ion currents through individual ion channels |
Studying ion channel function, electrophysiology |
High temporal resolution, direct measurement |
Technically challenging, invasive |
|
Single-Molecule FRET (smFRET) |
Measures energy transfer between two fluorophores |
Studying conformational changes, interactions |
Provides distance information, high sensitivity |
Requires labeling with fluorophores, photobleaching |
|
Electron Microscopy (EM) |
Uses electron beams to image structures at high resolution |
Structural biology, material science |
Very high resolution, detailed structural information |
Requires vacuum, sample preparation can be complex |
|
Nanopore Sequencing |
Detects changes in ionic current as molecules pass through a nanopore |
DNA sequencing, analyzing biomolecules |
Direct reading of sequence, long read lengths |
Limited read accuracy, complex data analysis |
Proteins
Structure of Proteins
Proteins have four levels of structure:
Types of Proteins
|
Protein |
Function |
Example |
|
Enzymes |
Catalyze biochemical reactions |
Amylase (digestion), DNA polymerase (replication) |
|
Structural |
Provide support and structure |
Collagen (connective tissue), Keratin (hair, nails) |
|
Transport |
Carry substances throughout the body |
Hemoglobin (oxygen transport), Albumin (nutrient transport) |
|
Motor |
Involved in movement |
Actin and Myosin (muscle contraction) |
|
Storage |
Store nutrients and other substances |
Ferritin (iron storage), Casein (milk protein) |
|
Signaling |
Coordinate bodily functions |
Insulin (regulates blood glucose levels), Hormones |
|
Receptor |
Receive and transmit signals |
G-protein coupled receptors (cell signaling) |
|
Defense |
Protect the body from pathogens |
Antibodies (immune response) |
Protein Synthesis
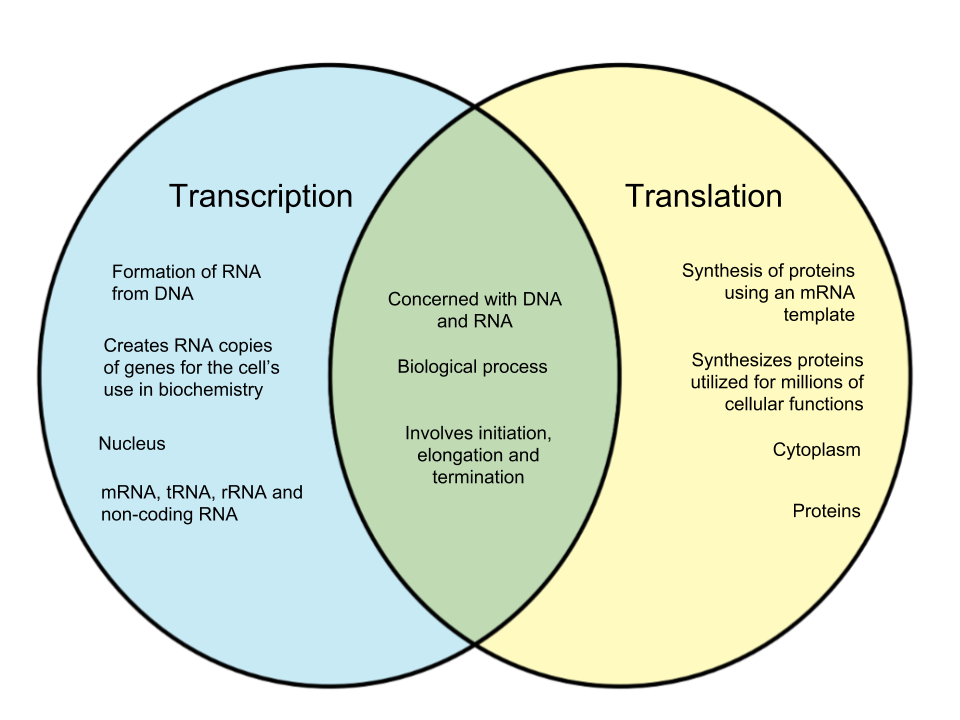
|
Protein Folding Protein folding is the physical process by which a protein chain acquires its native 3-dimensional structure, a conformation that is usually biologically functional. This process is crucial as the function of a protein is directly dependent on its structure. |
Functions of Proteins
Importance of Proteins in Nutrition
Protein-Related Disorders
Sources:
|
PRACTICE QUESTION Q: With reference to proteins, consider the following statements:
Which of the statements given above is/are correct? (a) 1 and 2 only (b) 2 and 3 only (c) 1 and 3 only (d) 1, 2, and 3 Answer: (a) |
© 2024 iasgyan. All right reserved