Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


Disclaimer: Copyright infringement not intended.
Context:
About Corbevax:
Vaccine Administration:
Development:
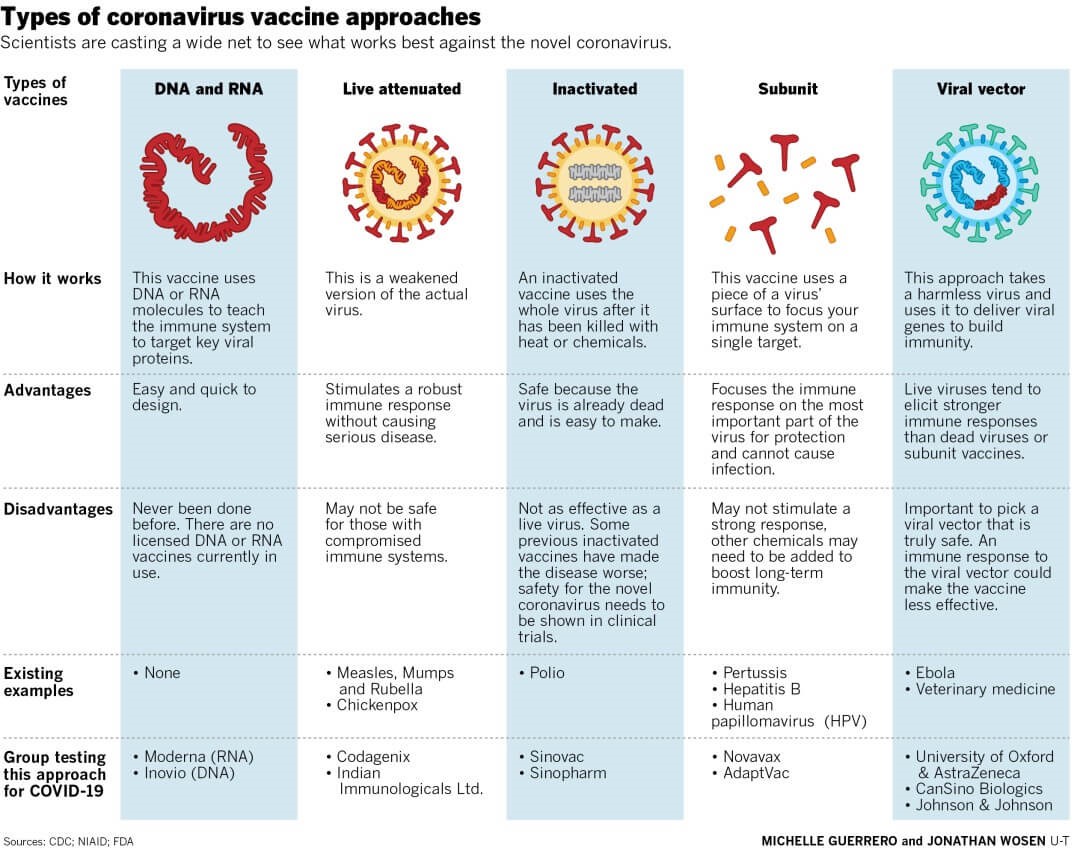
Types of Vaccine Platforms:
Whole Virus Vaccines:
https://www.pib.gov.in/PressReleasePage.aspx?PRID=1800918
© 2024 iasgyan. All right reserved