Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


Disclaimer: Copyright infringement not intended.
Context
Details
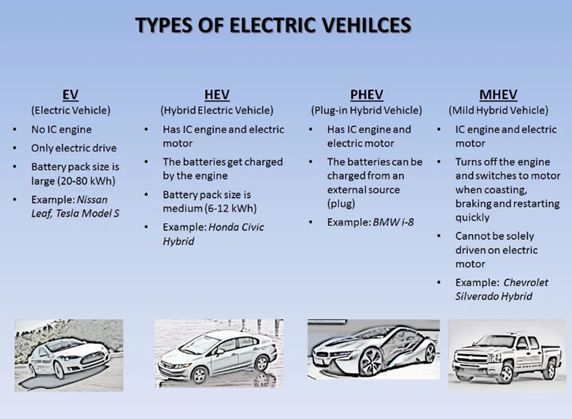
Electric Vehicles
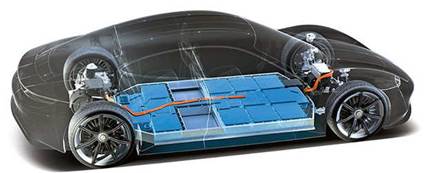
Electric vehicle battery
Battery
Types of Batteries used in automobiles
Lead-acid batteries
Nickel metal hydride batteries
Lithium-ion batteries
|
A variation on lithium-ion batteries, called lithium-ion polymer batteries, may also prove valuable to the future of EVs. These batteries may eventually cost less to build than lithium-ion batteries; however, at the present time, lithium-ion polymer batteries are prohibitively expensive. |
Ultracapacitors

Government Initiatives to promote EV

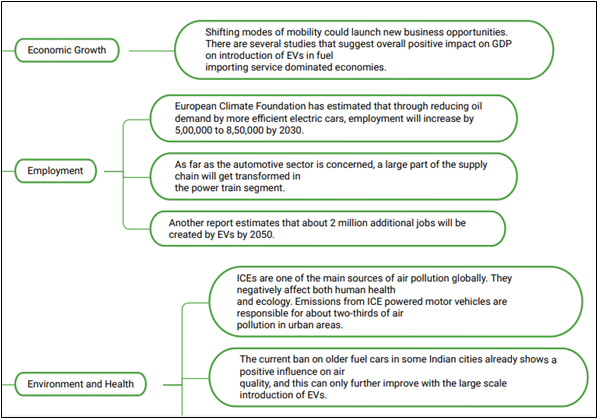
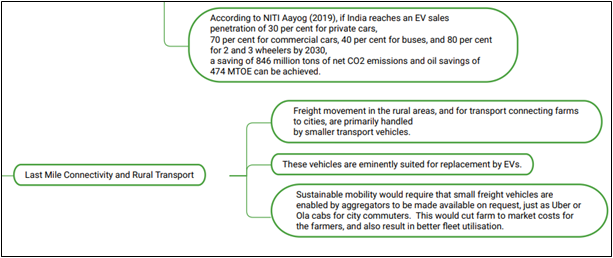
Impact of EVs

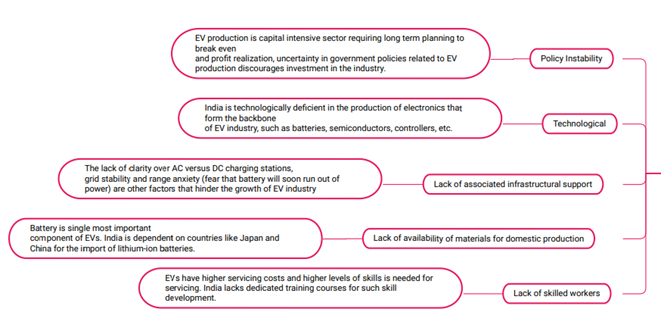
Challenges
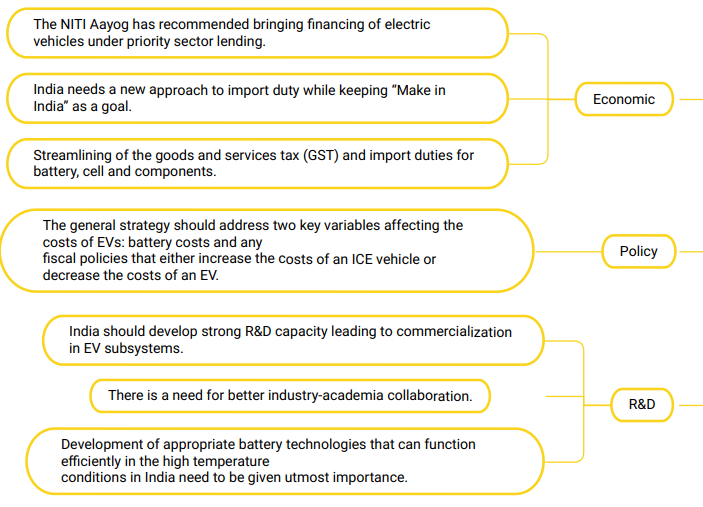
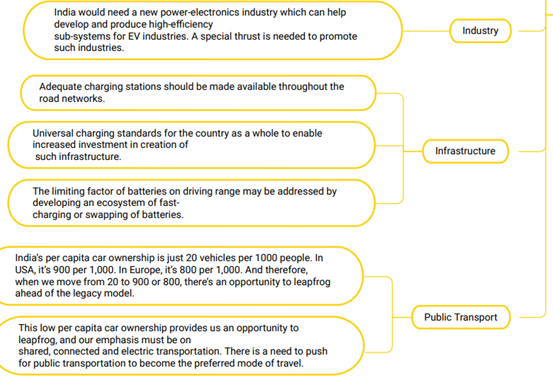
Way ahead
https://www.thehindu.com/business/Industry/gadkari-warns-of-heavy-penalties-and-vehicle-recalls-post-ev-explosion/article65342341.ece
© 2024 iasgyan. All right reserved