Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


Disclaimer: Copyright infringement not intended.
Context
Details
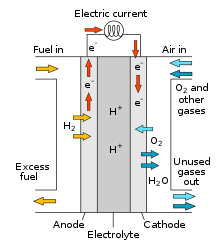
About Fuel Cells
How Fuel Cells Work:
Basic Components:
Fuel Sources:
Types of Fuel Cells:
Advantages of Fuel Cells:
Challenges:
Conclusion
Fuel cells hold tremendous potential as clean and efficient energy conversion devices, with ongoing research focusing on improving their performance, reducing costs, and expanding their applicability across industries and transportation sectors. Continued advancements in materials, manufacturing techniques, and infrastructure will play a pivotal role in their widespread adoption and commercialization.
|
PRACTICE QUESTION Q. How do fuel cells offer a viable solution in addressing energy needs while mitigating environmental impacts? Discuss with relevant examples. (250 Words) |
© 2024 iasgyan. All right reserved