Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


Disclaimer: Copyright infringement not intended.
Context
Details
About Halogens
Properties of Halogens:
Uses of Halogens:
Reactions of Halogens:
Significance of Halogens:
Conclusion
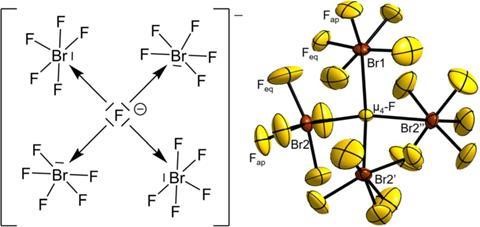
The synthesis and structural analysis of [NMe4][Br4F21]·BrF5 represent a remarkable achievement in inorganic chemistry, shedding light on novel bonding arrangements and pushing the boundaries of our understanding of interhalogen compounds.
|
PRACTICE QUESTION Q. Halogens are a diverse group of elements with unique properties and versatile applications across various industries. Comment. (15 marks) |
© 2024 iasgyan. All right reserved