Disclaimer: Copyright infringement not intended.
Context
- A new ozone hole has been detected over the tropics, at latitudes of 30 degrees South to 30 degrees North, a recent study claimed.
Ozone
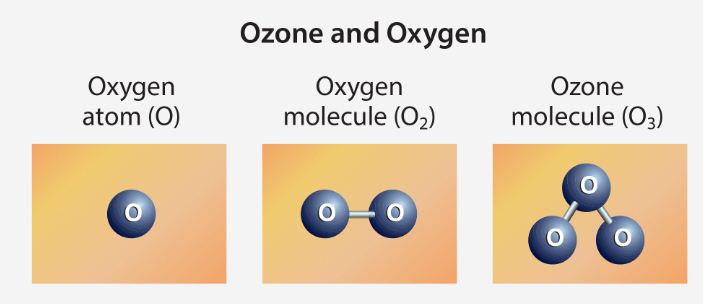
- Ozone is a molecule containing three oxygen atoms. It is blue in color and has a strong odor. Normal oxygen, which we breathe, has two oxygen atoms and is colorless and odorless.
- The Ozone Layer was discovered by the French physicists Charles Fabry and Henri Buisson in 1913.
How is Ozone created?
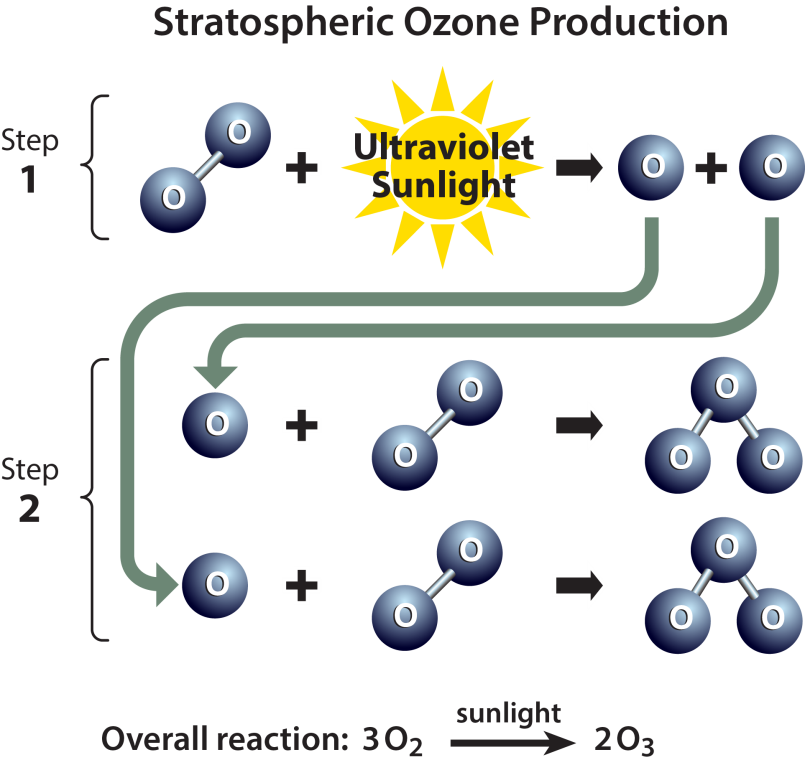
- When the sun's rays split oxygen molecules into single atoms, Ozone is created in the atmosphere. These single atoms combine with nearby oxygen to form a three-oxygen molecule — Ozone.
Why is Ozone Layer important?
- Ozone protects the Earth from harmful ultraviolet (UV) rays from the Sun. Without the Ozone layer in the atmosphere, life on Earth would be very difficult. Plants cannot live and grow in heavy ultraviolet radiation, nor can the planktons that serve as food for most of the ocean life.
- With a weakening of the Ozone Layer shield, humans would be more susceptible to skin cancer, cataracts and impaired immune systems.
Is Ozone harmful?
- Ozone can both protect and harm the Earth — it all depends on where it resides. For instance, if Ozone is present in the stratosphere of the atmosphere, it will act as a shield.
- However, if it is in the troposphere (about 10 km from the Earth's surface), Ozone is harmful. It is a pollutant that can cause damage to lung tissues and plants. Hence, an upset in the ozone balance can have serious consequences.
Ozone layers
Upper atmosphere
- Ozone occurs naturally in small (trace) amounts in the upper atmosphere (the stratosphere).
- Ozone protects life on Earth from the Sun’s ultraviolet (UV) radiation.
Lower atmosphere
- In the lower atmosphere (the troposphere) near the Earth’s surface, ozone is created by chemical reactions between air pollutants from vehicle exhaust, gasoline vapors, and other emissions. At ground level, high concentrations of ozone are toxic to people and plants.
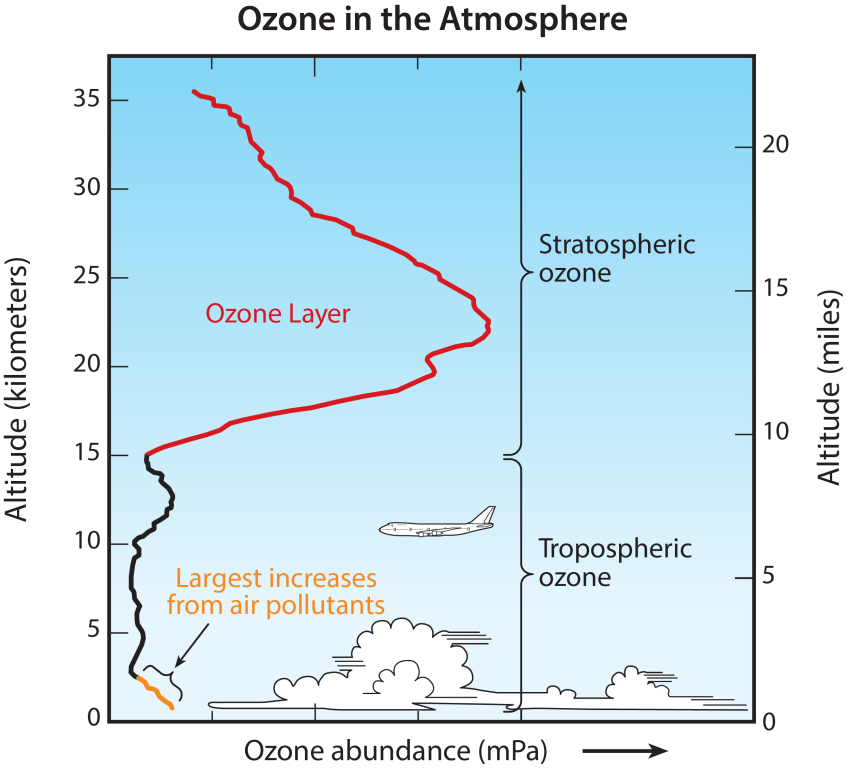
Stratospheric “good” ozone
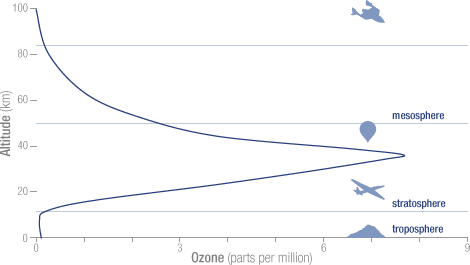
- Ninety percent of the ozone in the atmosphere sits in the stratosphere, the layer of atmosphere between about 10 and 50 kilometers altitude.
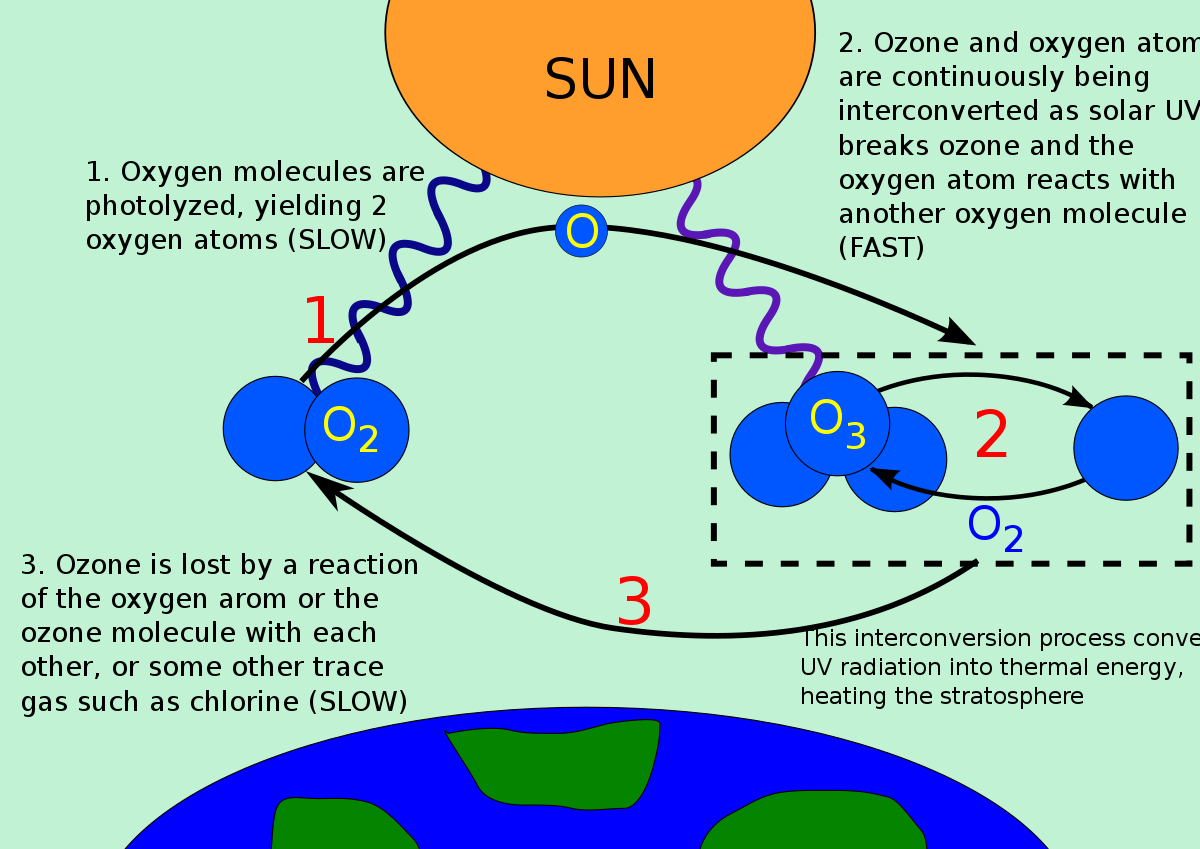
- The natural level of ozone in the stratosphere is a result of a balance between sunlight that creates ozone and chemical reactions that destroy it.
- Ozone is created when the kind of oxygen we breathe—O2—is split apart by sunlight into single oxygen atoms. Single oxygen atoms can re-join to make O2, or they can join with O2 molecules to make ozone (O3).
- The peak concentration of ozone occurs at an altitude of roughly 32 kilometers above the surface of the Earth. At that altitude, ozone concentration can be as high as 15 parts per million (0.0015 percent).
- Ozone is destroyed when it reacts with molecules containing nitrogen, hydrogen, chlorine, or bromine. Some of the molecules that destroy ozone occur naturally, but human activities have created others.
The concentration of ozone varies with altitude. Peak concentrations, an average of 8 molecules of ozone per million molecules in the atmosphere, occur between an altitude of 30 and 35 kilometers.
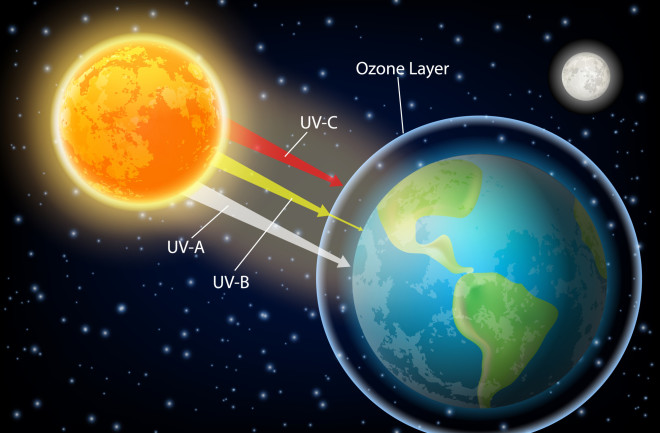
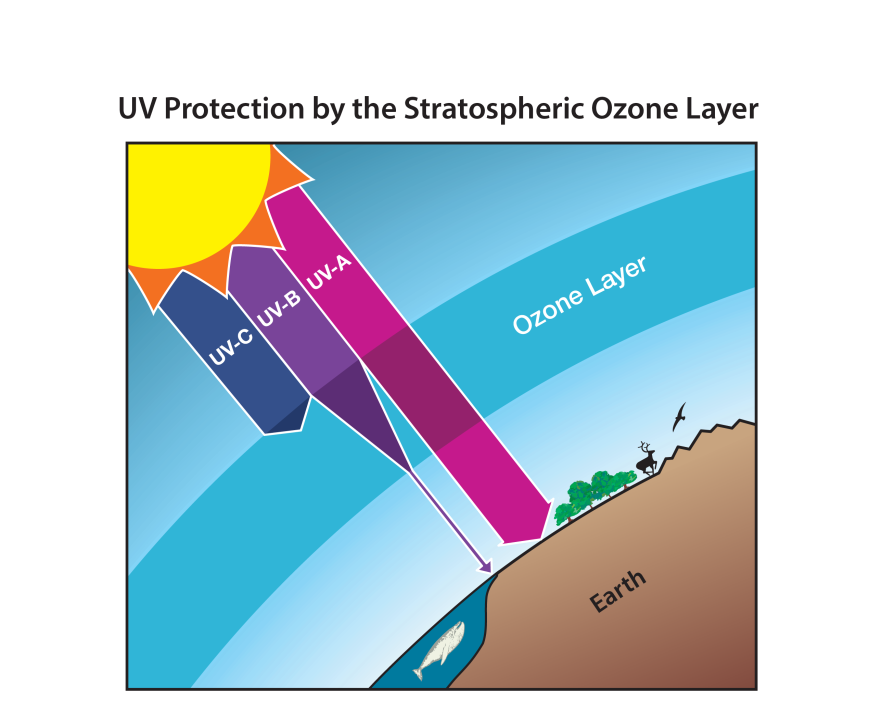
- Ozone in the stratosphere absorbs most of the ultraviolet radiation from the Sun. Without ozone, the Sun’s intense UV radiation would sterilize the Earth’s surface.
- Ozone screens all of the most energetic, UV-c, radiation, and most of the UV-b radiation.
- Ozone only screens about half of the UV-a radiation. Excessive UV-b and UV-a radiation can cause sunburn and can lead to skin cancer and eye damage.
- Increased levels of human-produced gases such as CFCs (chlorofluorocarbons) have led to increased rates of ozone destruction, upsetting the natural balance of ozone and leading to reduced stratospheric ozone levels. These reduced ozone levels have increased the amount of harmful ultraviolet radiation reaching the Earth’s surface. When scientists talk about the ozone hole, they are talking about the destruction of stratospheric, “good,” ozone.
Tropospheric “bad” ozone
- Although ozone high up in the stratosphere provides a shield to protect life on Earth, direct contact with ozone is harmful to both plants and animals (including humans).
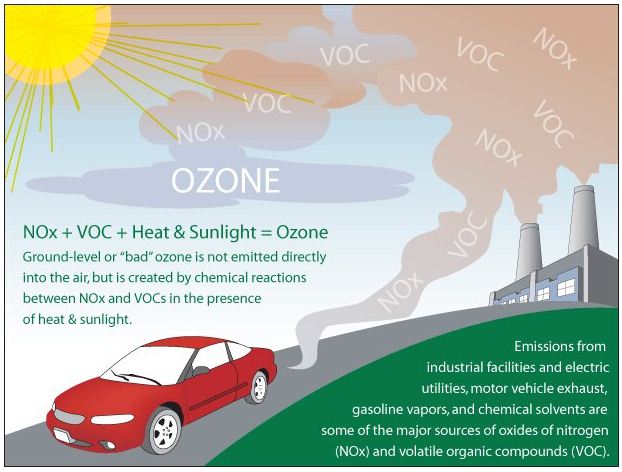
- Ground-level, “bad,” ozone forms when nitrogen oxide gases from vehicle and industrial emissions react with volatile organic compounds (carbon-containing chemicals that evaporate easily into the air, such as paint thinners) in presence of sunlight.
- In the troposphere near the Earth’s surface, the natural concentration of ozone is about 10 parts per billion (0.000001 percent). According to the Environmental Protection Agency, exposure to ozone levels of greater than 70 parts per billion for 8 hours or longer is unhealthy. Such concentrations occur in or near cities during periods where the atmosphere is warm and stable. The harmful effects can include throat and lung irritation or aggravation of asthma or emphysema.
Disruption of Ozone Balance in the atmosphere
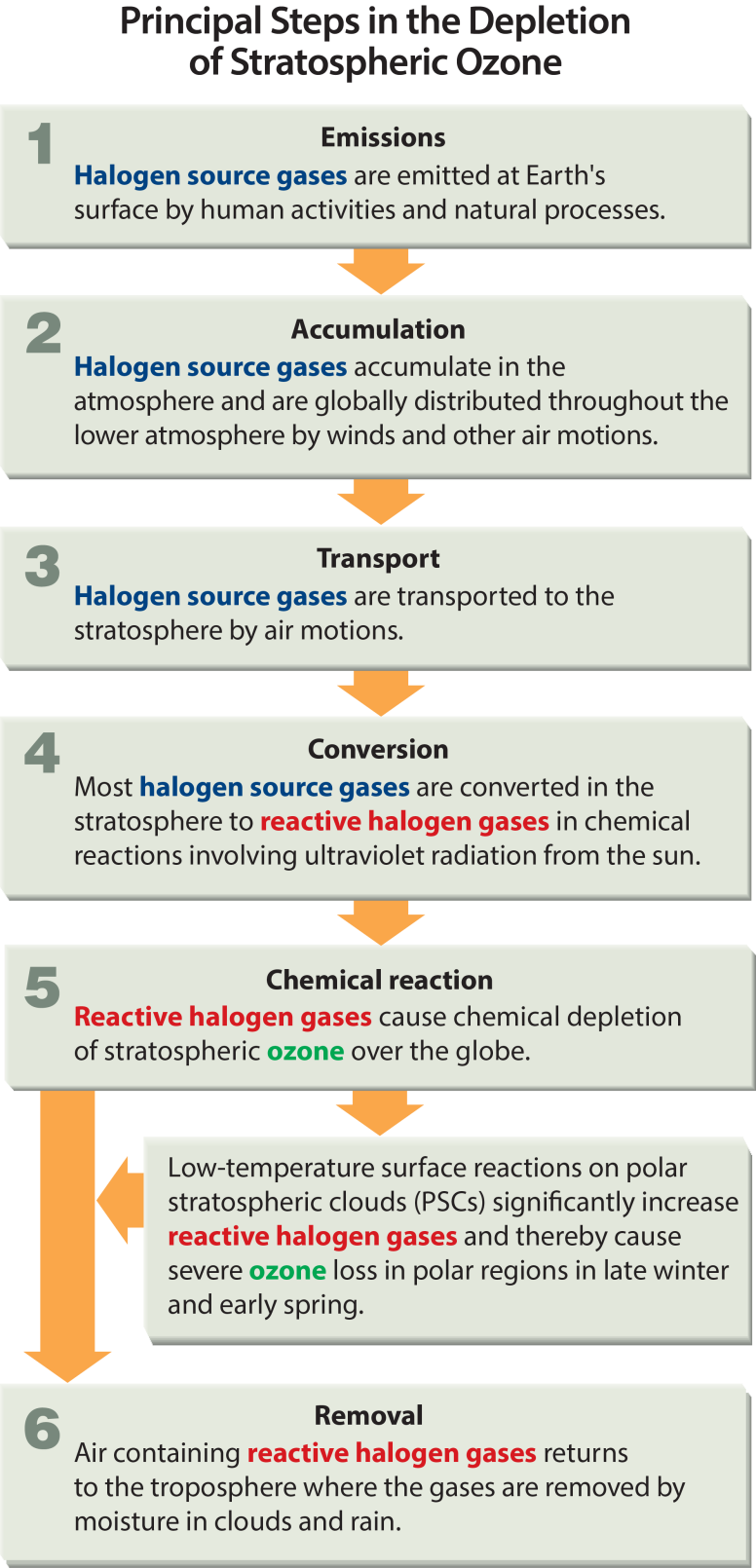
- Since the 1970s scientists have observed human activities to be disrupting the ozone balance. Production of chlorine-containing chemicals, such as chlorofluorocarbons (CFCs), has added to depletion of the Ozone Layer.
What is 'Ozone Layer depletion'?
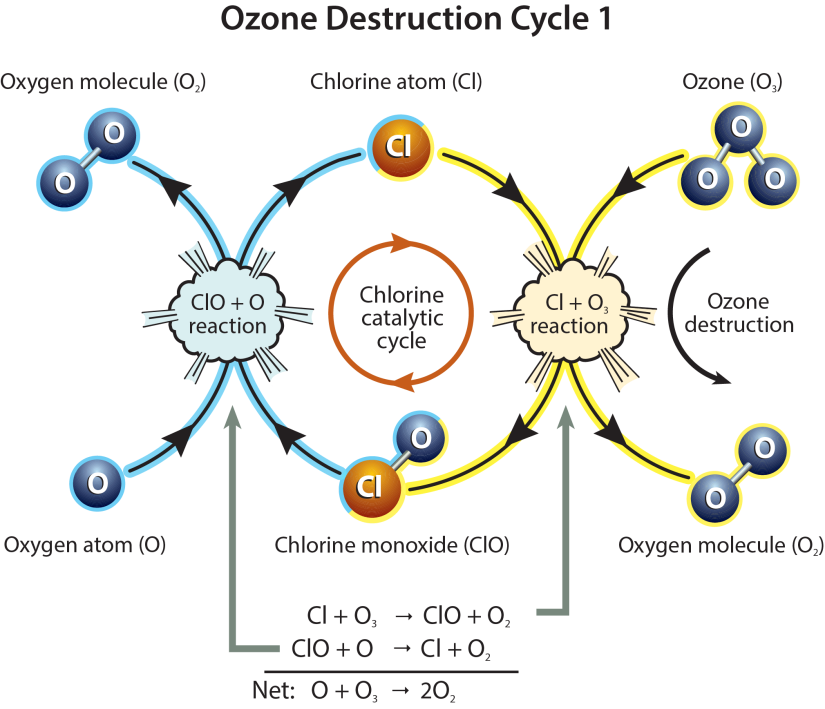
- When chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more quickly than it is naturally created.
- Ozone depletion is caused by the release of chlorofluorocarbons (CFCs) and other ozone-depleting substances (ODS), which were used widely as refrigerants, insulating foams, and solvents.
- Other chemicals that damage the ozone layer include methyl bromide (used as a pesticide) and halons (used in fire extinguishers). As methyl bromide and halons are broken apart, they release bromine atoms, which are 40 times more destructive to ozone molecules than chlorine atoms
- Depletion of the Ozone Layer occurs globally, but the severe depletion of the Ozone Layer over the Antarctic is often referred to as the 'Ozone Hole'. Increased depletion has recently started occurring over the Arctic as well.
Ozone depleting substances include:
- Chlorofluorocarbons (cfcs)
- Hydrochlorofluorocarbons (hcfcs)
- Hydrobromoflurocarbons (hbfcs)
- Halons
- Methyl bromide
- Carbon tetrachloride
- Methyl chloroform.
- Freons.
They have been used as:
- Refrigerants in commercial, home and vehicle air conditioners and refrigerators
- Foam blowing agents
- Components in electrical equipment
- Industrial solvents
- Solvents for cleaning (including dry cleaning)
- Aerosol spray propellants
- Fumigants.
Effects of Ozone Depletion
- Increased level of UV radiation
- Carcinoma
- Melanoma
- Effects on human health- sunburns, cataracts, aging or also to a weak immune system
- Increased tropospheric ozone- leads to health issues.
- Increased vitamin D production- can cause severe health conditions and can also increase the probability of mortality.
- Change in biogeochemical cycles- can alter sources and sinks of greenhouse gases and thus can indirectly contribute to the global warming issue.
- Effects on marine life- can harm the growth of plankton. A decrease in plankton will therefore lead to a disruption of the whole marine food chain.
- Effects on animals- animals can also suffer from skin cancer and additional diseases caused by UVB radiation.
- Effects on plants- can have an adverse effect on the growth of plants.
- Effects on crops- UVB radiation is known to be able to change parts of the plant’s DNA. This may lead to reduced crop yields or other issues related to it.
- Environmental impact- without ozone layer, the whole food chain would collapse within a few days or weeks.
- Economic impact- lower crop yields and other harmful effects is likely to imply serious financial downsides on a global scale.
How do we know that natural sources are not responsible for ozone depletion?
- While it is true that volcanoes and oceans release large amounts of chlorine, the chlorine from these sources is easily dissolved in water and washes out of the atmosphere in rain.
- In contrast, CFCs are not broken down in the lower atmosphere and do not dissolve in water. The chlorine in these human-made molecules does reach the stratosphere. The increase in stratospheric chlorine since 1985 matches the amount released from CFCs and other ozone-depleting substances produced and released by human activities.
What is being done about ozone depletion?
- In 1978, the use of CFC propellants in spray cans was banned in the U.S. In the 1980s, the Antarctic “ozone hole” appeared and an international science assessment more strongly linked the release of CFCs and ozone depletion.
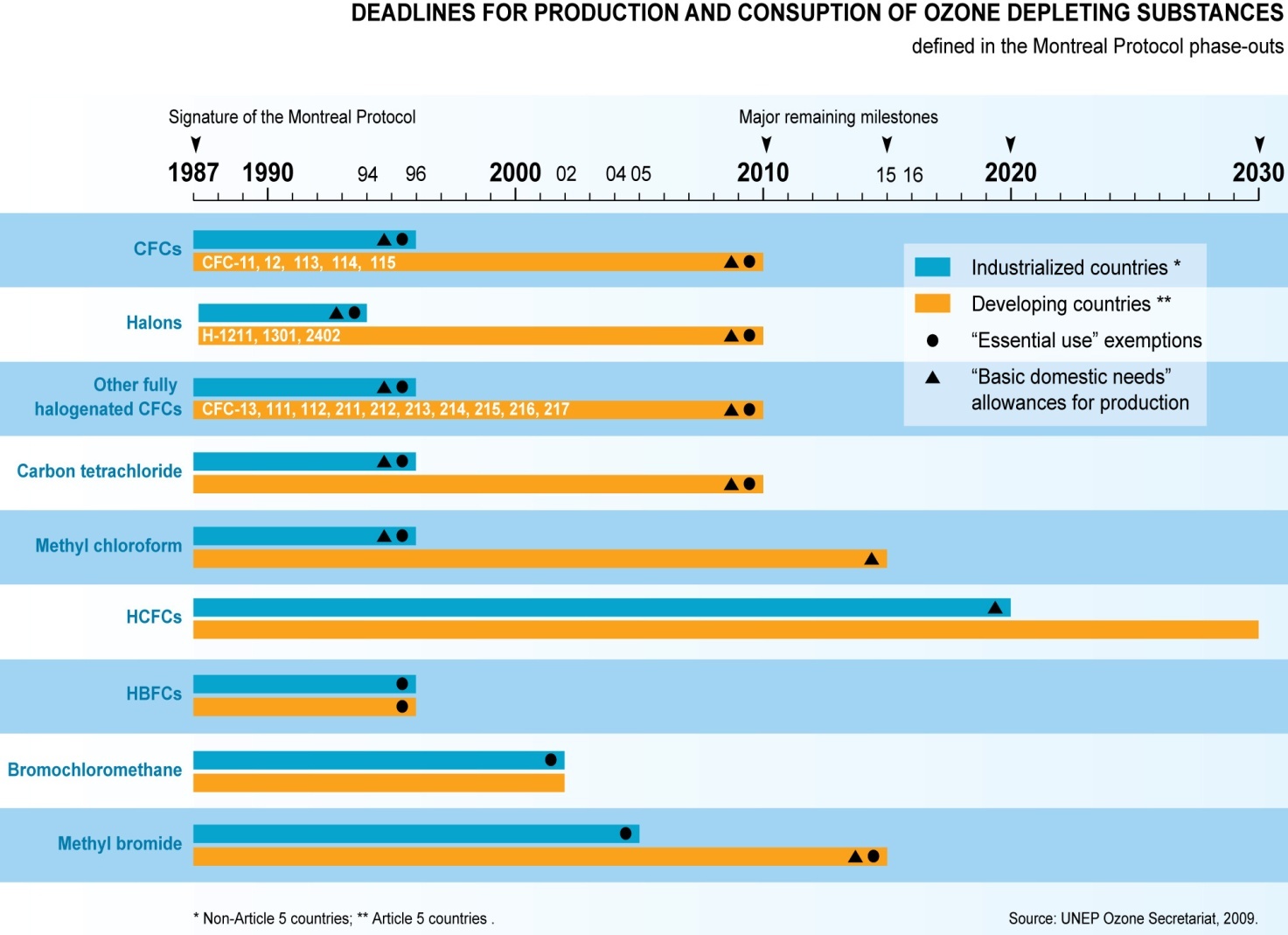
- In 1987, the Montreal Protocol was signed and the signatory nations committed themselves to a reduction in the use of CFCs and other ozone-depleting substances.
Montreal Protocol on Ozone Layer depletion
- Montreal Protocol is a multilateral environmental agreement that regulates the production and consumption of ozone-depleting substances (ODS). It was adopted on September 15, 1987.
- Since 1987, the treaty has been amended to ban CFC production after 1995 in the developed countries, and later in developing.
India has phased out chlorofluorocarbons, carbon tetrachloride, halons, methyl bromide and methyl chloroform for controlled uses in line with the Montreal Protocol.
India has successfully achieved the complete phase out of Hydrochlorofluorocarbon (HCFC)-141 b, which is a chemical used by foam manufacturing enterprises and one of the most potent ozone depleting chemical after Chlorofluorocarbons (CFCs).
Vienna Convention
- The Vienna Convention for the Protection of the Ozone Layer is a multilateral environmental agreement signed in 1985 that provided frameworks for international reductions in the production of chlorofluorocarbons due to their contribution to the destruction of the ozone layer, resulting in an increased threat of skin cancer.
- The Vienna Convention was agreed upon at the Vienna Conference of 1985 and entered into force in 1988. The Vienna Convention provided the framework necessary to create regulatory measures in the form of the Montreal Protocol.
- While not a binding agreement, it acts as a framework for the international efforts to protect the ozone layer; however, it does not include legally binding reduction goals for the use of CFCs, the main chemical agents causing ozone depletion.
Kigali Amendment to the Montreal Protocol
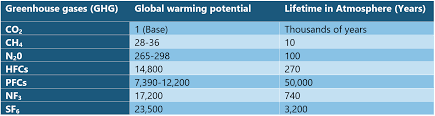
- The 2016 Kigali Amendment to the Montreal Protocol is an international agreement to gradually reduce the consumption and production of hydrofluorocarbons (HFCs).
- Countries agreed to add HFCs to the list of controlled substances, and approved a timeline for their gradual reduction by 80-85 per cent by the late 2040s.
- It is a legally binding agreement designed to create rights and obligations in international law.
Note: HFCs were used to replace the substances banned in Montreal agreement because they have zero impact on the ozone. However, HFCs are powerful greenhouse gases that contribute to global warming and climate change, so this amendment adds HFCs to the list of chemicals that countries promise to phase down.
India will complete its phase down of HFCs in 4 steps from 2032 onwards with cumulative reduction of 10% in 2032, 20% in 2037, 30% in 2042 and 85% in 2047.
Has the Montreal Protocol been successful?
- The Montreal Protocol has been successful in slowing and reversing the increase of ozone-depleting gases (halogen source gases) in the atmosphere. An important measure of its success is the change in the value of effective stratospheric chlorine.
- With the full and sustained implementation of the Montreal Protocol, the ozone layer is projected to recover by the middle of this century. Without this treaty, ozone depletion would have increased tenfold by 2050 compared to current levels, and resulted in millions of additional cases of melanoma, other cancers and eye cataracts. It has been estimated, for example, that the Montreal Protocol is saving an estimated two million people each year by 2030 from skin cancer.
- To date, the Parties to the Protocol have phased out 98% of ODS globally compared to 1990 levels. Because most of these substances are potent greenhouse gases, the Montreal Protocol is also contributing significantly to the protection of the global climate system. From 1990 to 2010, the treaty’s control measures are estimated to have reduced greenhouse gas emissions by the equivalent of 135 gigatons of CO2, the equivalent of 11 gigatons a year.

Steps to control ozone layer depletion:
Controlling ozone layer depletion can be done in the following ways.
- Every individual can help reduce ozone layer depletion by taking public transportation, buying recycled products, conserving electricity, etc.
- Refrigerators and air conditioners that do not use CFCs as a refrigerant can be used.
- Preference should be given to those aerosols that do not contain CFCs or
- When repairing air conditioners or refrigerators, care should be taken by transferring the refrigerant to a recycling machine rather than exposing it to the atmosphere.
- Spreading public awareness about the causes, impacts, and controlling measures of ozone layer depletion.
- Since this is not a problem limited to a single country, international agreements to restrict CFC emissions from industries can be developed.
https://www.downtoearth.org.in/news/climate-change/does-tropical-ozone-hole-exist-experts-are-divided-83745
















