Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


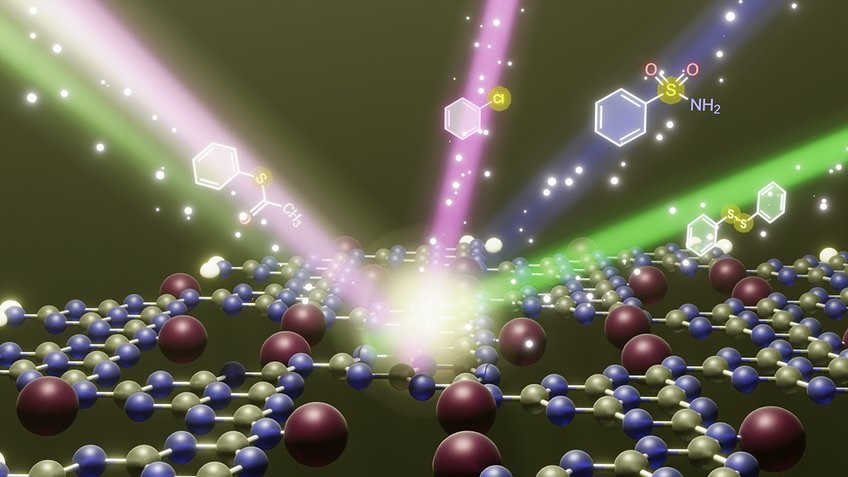
Disclaimer: Copyright infringement not intended.
Context
Details
UC-POP-Au Photocatalyst
Introduction to Photocatalysis
Working Principles:
Types of Photocatalysts:
Applications of Photocatalysts:
Introduction to Chemical Warfare Agents
Classification of Chemical Warfare Agents:
Effects on Human Health:
Historical Use:
International Conventions and Treaties:
Introduction to Mustard Gas
Properties and Mechanism of Action:
Effects on Human Health:
Conclusion
In summary, the groundbreaking UC-POP-Au photocatalyst developed by IISER Bhopal opens new dimensions in photocatalysis by harnessing sunlight to combat chemical threats, offering potential solutions for environmental and security challenges.
|
PRACTICE QUESTION Q. Elaborate on the significance of the UC-POP-Au photocatalyst developed by IISER Bhopal researchers in the context of chemical warfare agent neutralization. (250 Words) |
© 2024 iasgyan. All right reserved