Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now


Disclaimer: Copyright infringement not intended.
Context
Archaeologists have used radiocarbon dating to analyze the oldest true wooden frame saddle in East Asia, revealing how the rise of Mongolian steppe cultures was likely aided by advances in equestrian technology.
Details
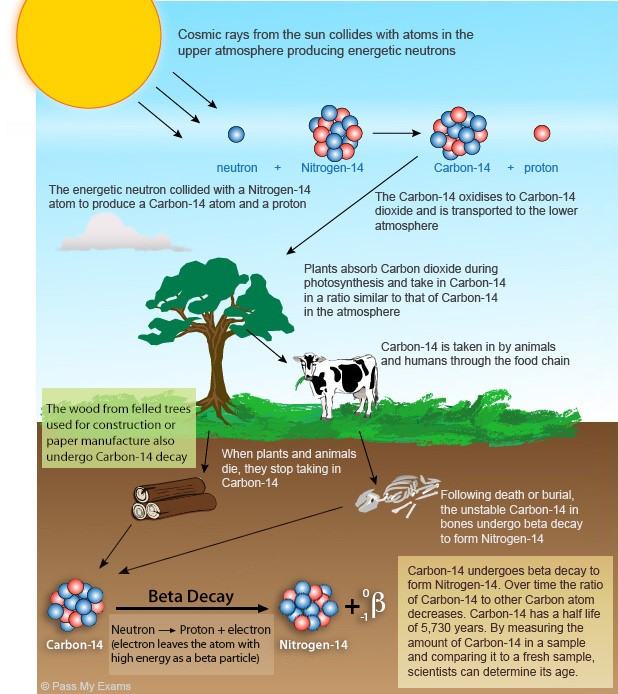
What is Carbon-14?
Radioactive Decay of Carbon-14:
Dating Process
Limitations and Considerations
Applications
Conclusion
Radiocarbon dating provides invaluable insights into the age of organic materials and has significantly contributed to our understanding of human history and the natural world. Despite its limitations, it remains a powerful tool in scientific research. Continuous advancements in technology and calibration methods enhance its accuracy and broaden its applications across various disciplines.
|
PRACTICE QUESTION Q. Discuss the significance of radiocarbon dating in determining the chronology of archaeological sites and its contribution to understanding human history. Highlight its limitations and the advancements in the field that have enhanced its accuracy. (250 Words) |
© 2024 iasgyan. All right reserved