Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now



Copyright infringement not intended
Picture Courtesy: www.hindustantimes.com
Context: The Union Health Ministry notifies revised rules under Schedule M of the Drugs and Cosmetics Rules, 1945, aiming to align Indian guidelines with global standards and strengthen the quality of domestically manufactured drugs.
Details
Objective and Scope
Evolution of GMP
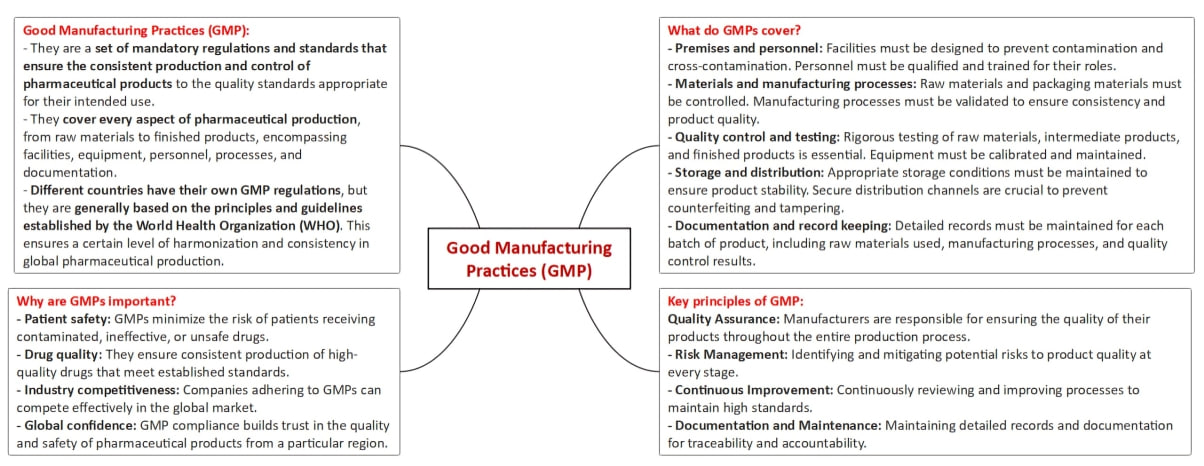
Alignment with Global Standards
Key Changes Introduced
New Categories of Drugs
Manufacturer Responsibilities
Testing and Quality Assurance
Implementation Timeline
Industry Response
Conclusion
Must Read Articles:
WHO Standard Good Manufacturing Practices: https://www.iasgyan.in/daily-current-affairs/who-standard-good-manufacturing-practices
|
PRACTICE QUESTION Q. How does the World Health Organization influence and regulate the global healthcare system, and what key roles does it play in ensuring international health standards and coordination among member nations? |
© 2024 iasgyan. All right reserved