Free Courses Sale ends Soon, Get It Now


Free Courses Sale ends Soon, Get It Now



Disclaimer: Copyright infringement not intended.
Context
Background
Why does a new isotope matter?
The new Research Techniques
Multinucleon Transfer
Time-of-Flight Mass Spectrometry
Findings
Significance
Trivia
Isotopes
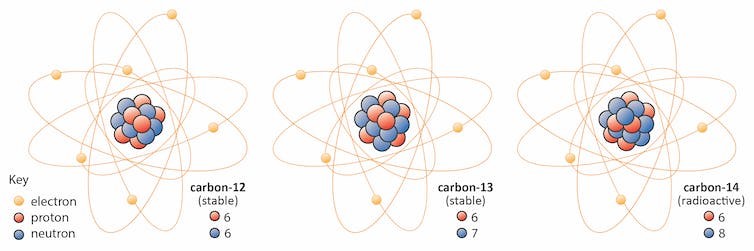
Origin of Isotopes
Isotope Facts
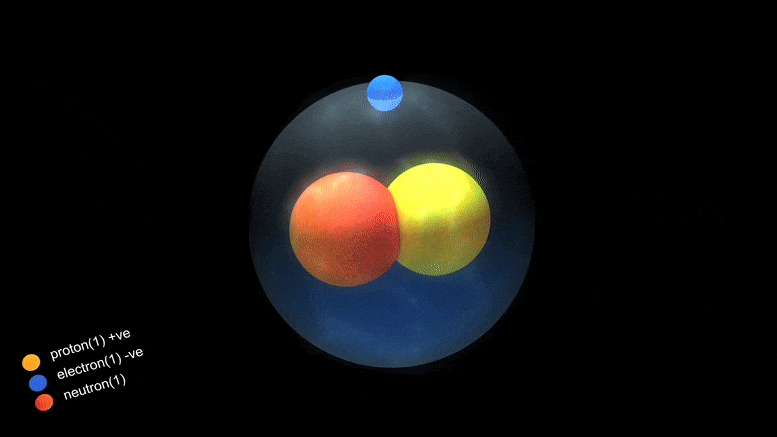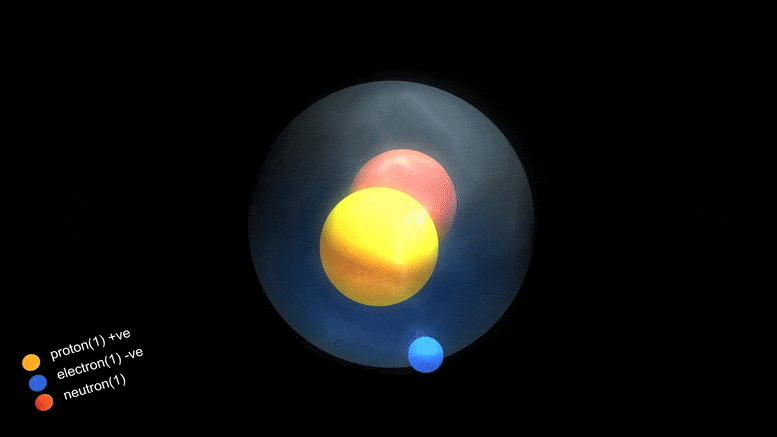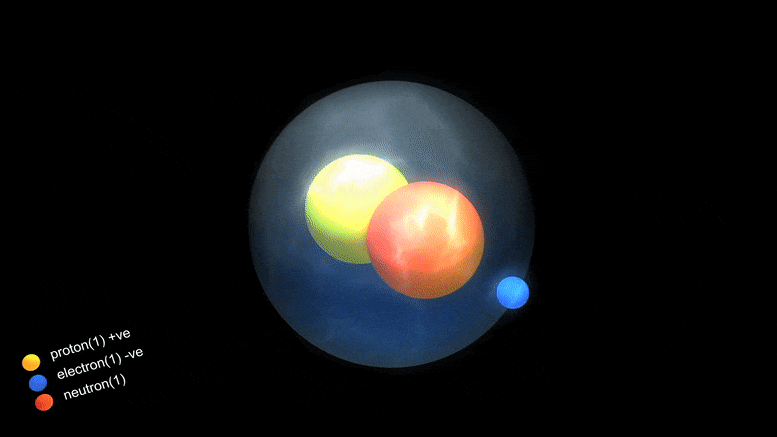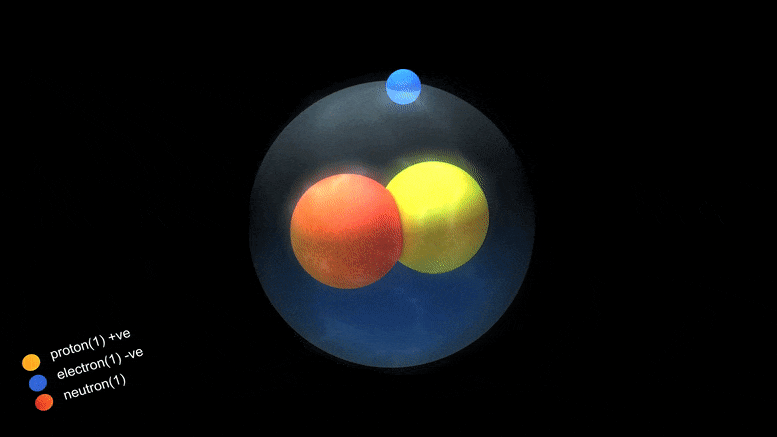
‘Magic Numbers’
|
PRACTICE QUESTION Q. Which of the following statements are correct in reference to Isotopes? a) Multinucleon transfer is a phenomenon, in which isotopes swap neutrons and protons. b) All artificial isotopes are unstable and therefore radioactive; scientists call them radioisotopes. c) Carbon-12 is a stable isotope, meaning it never undergoes radioactive decay. d) Elements can have both stable and radioactive isotopes. 1. a and b 2. b and d 3. a, c and d. 4. All of the above. Correct Answer: Option 4 |
© 2024 iasgyan. All right reserved